Your Internal Clock, Sleep and Blue Light
Melatonin, Cancer, with a touch of Depression
It was brisk outside as I pulled up to the harbor to enjoy the ocean for a few minutes prior to going into my meeting. I find it relaxing and peaceful. However, not long ago as I was walking along a nicely dressed man walked up to me, greeted me and asked, “What time of day is it?” I do not wear a watch but seems nearly everyone these days has a cell phone. I glanced down and answered “it’s ten ‘til 7”
He clarified his question. “Is it morning or evening?” “It’s morning I answered”, I was very puzzled by the question thinking it obvious it was morning.
He seemed a bit irritated and under his breath said “damn it” and then went on his way.
I wondered to myself how could anyone confuse morning with evening. As I looked into it more I found out more about our internal clock and how we can determine by light what time of day it is and how it affects our sleep. In my research I found some very interesting correlations with light, hormones in the body, particularly Melatonin, a hormone produced in the Pineal gland of the body, and sleep.
Ever since the 1850s when Mueller identified the rods and the cones in the retina of the eye as photoreceptors, it was believed that these were the only light sensing receptors in the eye. This doctrine collapsed in 2001 as a result of work done by Debra Skene.
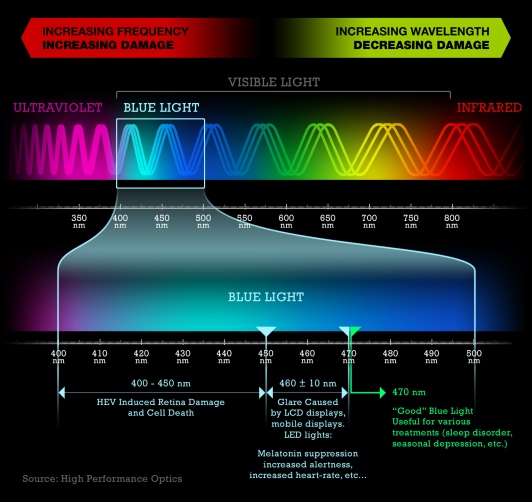
Skene and her colleagues at the University of Surrey tested whether some wavelengths of light had greater effect than others at suppressing melatonin. They shone light into 22 people’s eyes in the middle of the night, when melatonin levels are highest. Melatonin production was clearly sensitive to light and less was made as light intensity increased. Testing the relative effect of different wavelengths of light produced a surprising result. The shortest wavelengths, what we see as dark blue, caused the greatest drop in melatonin.
In addition to the rods and cones which we are familiar with and do not pick up much of this end of the light spectrum, it was speculated that there was another type of photoreceptor which was able to detect this wavelength of light.
The following year, David Berson of Brown University successfully isolated these new photoreceptors and described them as a type of retinal ganglion cell. These cells extend long projections into a part of the brain called suprachiasmatic nucleus (SCN) so they can transmit information directly from the eyes. This is the region of the brain that regulates the circadian clock, the body’s internal timer that tells all the organs and even individual cells what ‘time’ it is.
Berson originally isolated these cells in animals but correctly predicted that they play the same role in people, controlling release of melatonin.
In 2003, Lockley et al confirmed that these shorter wavelength of light have a greater effect. The short 460 nm wavelengths of light suppress melatonin twice as much as longer 555 nm light, the wavelength best seen by the human eye.
This is useful knowledge. Ever since Edison invented the light bulb, we have inadvertently been offsetting our internal clocks by using artificial lighting. Controlling which wavelengths of light reach the eye gives us a way to reset and control our internal clocks. It has also opened up the possibility of simple treatments for some unexpected conditions.
In 2005 Christian Cajochen of the Basel, Switzerland reported his success at resetting the circadian clock of test subjects using colored lights. He and his colleagues had nine male volunteers spend an evening and night in a room in which the researchers controlled the lights. In separate rounds, these volunteers were exposed to indigo-blue light with a wavelength of 460 nanometers, green-yellow light with a wavelength of 550 nm, or complete darkness. Throughout the experiment, the researchers monitored each fellow’s daily sleep-wake cycle.
Volunteers in total darkness during the 2-hour period displayed normal nighttime trends, including reduced core-body temperatures, slower heart rates, elevated melatonin concentrations in saliva, and increased sleepiness.
Exposing them to blue light suppressed those changes, while green-yellow light had a minimal effect, the researchers reported in the March 2005 issue of the Journal of Clinical Endocrinology & Metabolism.
I think of this as I walk the dog at night and see the blue lit house windows illuminated from the television sets in the neighborhood. How much of our ability to watch late night TV is connected with that blue?
Some of the potential applications of this knowledge are obvious. Strengthening the circadian rhythm should help with insomnia. Avoiding suppression of melatonin by adjusting nighttime light frequencies could lower risk of certain cancers. Some applications are less obvious.
Teenagers and their peculiar sleep habits were one of the first applications I read about. Anyone who has lived with a teenager will testify that they have little no sense of when it is bed time and tend to stay up way too late. Experts say teens need 9 hours of sleep a night, but less than 20% get this much. The US National Sleep Foundation tells us that 25% of teens fall asleep in class at least once a week.
Mary Carskadon from Brown Medical School says that about half of the teenagers she tested actually show symptoms of narcolepsy. She found that many teens drop immediately into rapid eye movement (REM) sleep, skipping over the first stages of non-rapid eye movement sleep (NREM), a distinct symptom of narcolepsy.
We need light in the morning and then darkness in the evening to set our circadian clocks. Kids go straight to school after waking at times so early that much of the year it is still dark. They don’t see natural sun light until late in the day, if they do at all. A well-lit class room is illuminated at about 400 lux; compare that to the 10,000 lux experienced outdoors on a dull, rainy day or the than 100,000 lux experienced on a bright day. Is it any wonder they don’t know when to go to sleep?
The very intriguing paper I have come across so far using these blue lens is on bipolar disease. James Phelps writing in a recent issue of Medical Hypothesis combines this knowledge of the retinal ganglia with past research on what is called “dark therapy” for treating bipolar. In 1998 Wehr et al. suggested that, “Fostering sleep and stabilizing its timing by scheduling regular nightly periods of enforced bed rest in the dark may help to prevent mania and rapid cycling in bipolar patients.” A 1999 paper describes a case history in which this dark therapy used successfully to treat a rapidly cycling bipolar patient. A 2005 paper in Bipolar Disorders confirmed the idea, “that extended bed rest and darkness could stabilize mood swings in rapid cycling bipolar patients.”
Forcing patients to live in darkened rooms is apparently difficult outside of an in-patient setting. Phelps theorizes that a similar effect to ‘dark therapy’ can be achieved simply by wearing yellow tinted glasses that filter out blue light in the evening. He reports success with several patients.
Of course if filtering out blue light is useful for treating bipolar, a troubling question quickly arises. Could our increased evening exposure to blue light emanating from fluorescent lighting, televisions and computer screens account for the incidence of bipolar disorders in adults and especially in children? We have also assumed that the explanation of why certain disease incidence increases with latitude is less UV exposure and consequently a greater chance of vitamin D deficiency. Another possible explanation is that at higher latitudes, people depend on artificial lighting for more of the year.
Other suggested uses for these blue light filtering glasses include treating insomnia (of course), infertility and depression. Another condition, fibromyalgia also comes to mind because it as generally being accompanied by significant sleep disturbances. Though I’ve seen no published evidence yet, suggesting these blue blocking lenses for this patient group seems reasonable.
Everyone has slightly different circadian rhythms, but the average length is 24 and one-quarter hours. The circadian rhythm of people who stay up late is slightly longer, while the rhythms of earlier birds fall short of 24 hours. Dr. Charles Czeisler of Harvard Medical School showed, in 1981, that daylight keeps a person’s internal clock aligned with the environment.
Study after study has linked working the night shift and exposure to light at night to several types of cancer (breast, prostate), diabetes, heart disease, and obesity. It’s not exactly clear why nighttime light exposure seems to be so bad for us. But we do know that exposure to light suppresses the secretion of melatonin, a hormone that influences circadian rhythms, and there’s some experimental evidence (it’s very preliminary) that lower melatonin levels might explain the association with cancer.
A Harvard study shed a little bit of light on the possible connection to diabetes and possibly obesity. The researchers put 10 people on a schedule that gradually shifted the timing of their circadian rhythms. Their blood sugar levels increased, throwing them into a prediabetic state, and levels of leptin, a hormone that leaves people feeling full after a meal, went down.
Even dim light can interfere with a person’s circadian rhythm and melatonin secretion. A mere eight lux—a level of brightness exceeded by most table lamps and about twice that of a night light—has an effect, notes Stephen Lockley, a Harvard sleep researcher. Light at night is part of the reason so many people don’t get enough sleep, says Lockley, and researchers have linked short sleep to increased risk for depression, as well as diabetes and cardiovascular problems.
What you can do
- Use dim red lights for night lights. Red light has the least power to shift circadian rhythm and suppress melatonin.
- Avoid looking at bright screens beginning two to three hours before bed.
- If you work a night shift or use a lot of electronic devices at night, consider wearing blue-blocking glasses or installing an app that filters the blue/green wavelength at night.
- Expose yourself to lots of bright light during the day, which will boost your ability to sleep at night, as well as your mood and alertness during daylight.
Here’s to you and a better night’s sleep.
Yours for Better Health, Dr. Shapero
EXPECT MIRACLES – WE DO
www.premierhealthcaresc.com
References:
J Physiol. 2001 Aug 15;535(Pt 1):261-7.
An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans.
Thapan K, Arendt J, Skene DJ.
Centre for Chronobiology, School of Biomedical and Life Sciences, University of Surrey, Guildford, Surrey GU2 7XH, UK.
-
Non-image forming, irradiance-dependent responses mediated by the human eye include
synchronisation
of the circadian axis and suppression of pineal melatonin production. The retinal photopigment(s) transducing these light responses in humans have not been
characterised
. 2. Using the ability of light to suppress nocturnal melatonin production, we aimed to investigate its spectral sensitivity and produce an action spectrum. Melatonin suppression was quantified in 22 volunteers in 215 light exposure trials using monochromatic light (30 min pulse administered at circadian time (CT) 16-18) of different wavelengths (lambda(max) 424, 456, 472, 496, 520 and 548 nm) and irradiances (0.7-65.0 microW cm(-2)). 3. At each wavelength, suppression of plasma melatonin increased with increasing irradiance. Irradiance-response curves (IRCs) were fitted and the generated half-maximal responses (IR(50)) were corrected for lens filtering and used to construct an action spectrum. 4. The resulting action spectrum showed unique short-wavelength sensitivity very different from the classical scotopic and photopic visual systems. The lack of fit (r(2) < 0.1) of our action spectrum with the published rod and cone absorption spectra precluded these photoreceptors from having a major role. Cryptochromes 1 and 2 also had a poor fit to the data. Fitting a series of Dartnall nomograms generated for rhodopsin-based photopigments over the lambda(max) range 420-480 nm showed that rhodopsin templates between lambda(max) 457 and 462 nm fitted the data well (r(2) > or =0.73). Of these, the best fit was to the rhodopsin template with lambda(max) 459 nm (r(2) = 0.74). 5. Our data strongly support a primary role for a novel short-wavelength photopigment in light-induced melatonin suppression and provide the first direct evidence of a non-rod, non-cone photoreceptive system in humans.
PMID: 11507175 [PubMed – indexed for MEDLINE]
issue 2330 of New Scientist magazine, 16 February 2002, page 17
Trends Neurosci. 2003 Jun;26(6):314-20.
Strange vision: ganglion cells as circadian photoreceptors.
Berson DM.
Department of Neuroscience, Brown University, Providence, RI 02912, USA. david_berson@brown.edu
A novel photoreceptor of the mammalian retina has recently been discovered and characterized. The novel cells differ radically from the classical rod and cone photoreceptors. They use a unique photopigment, most probably melanopsin. They have lower sensitivity and spatiotemporal resolution than rods or cones and they seem specialized to encode ambient light intensity. Most surprisingly, they are ganglion cells and, thus, communicate directly with the brain. These intrinsically photosensitive retinal ganglion cells (ipRGCs) help to synchronize circadian rhythms with the solar day. They also contribute to the pupillary light reflex and other behavioral and physiological responses to environmental illumination.
Chronobiol Int. 2004 Mar;21(2):189-204.
J Clin Endocrinol Metab. 2003 Sep;88(9):4502-5.
High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light.
Lockley SW, Brainard GC, Czeisler CA.
Division of Sleep Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, Massachusetts 02115, USA.
The endogenous circadian oscillator in mammals, situated in the suprachiasmatic nuclei, receives environmental photic input from specialized subsets of photoreceptive retinal ganglion cells. The human circadian pacemaker is exquisitely sensitive to ocular light exposure, even in some people who are otherwise totally blind. The magnitude of the resetting response to white light depends on the timing, intensity, duration, number and pattern of exposures. We report here that the circadian resetting response in humans, as measured by the pineal melatonin rhythm, is also wavelength dependent. Exposure to 6.5 h of monochromatic light at 460 nm induces a two-fold greater circadian phase delay than 6.5 h of 555 nm monochromatic light of equal photon density. Similarly, 460 nm monochromatic light causes twice the amount of melatonin suppression compared to 555 nm monochromatic light, and is dependent on the duration of exposure in addition to wavelength. These studies demonstrate that the peak of sensitivity of the human circadian pacemaker to light is blue-shifted relative to the three-cone visual photopic system, the sensitivity of which peaks at approximately 555 nm. Thus photopic lux, the standard unit of illuminance, is inappropriate when quantifying the photic drive required to reset the human circadian pacemaker.
Cajochen, C., et al. 2005. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. Journal of Clinical Endocrinology & Metabolism 90(March):1311-1316. Abstract available at http://jcem.endojournals.org/cgi/content/abstract/90/3/1311. Full article available at http://www.chronobiology.ch/pdf/CC_lightpaper.pdf.
J Clin Endocrinol Metab. 2005 Mar;90(3):1311-6. Epub 2004 Dec 7.
High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light.
Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, Orgül S, Wirz-Justice A.
Centre for Chronobiology, Psychiatric University Clinic, Wilhelm Kleinstr. 27, CH-4025 Basel, Switzerland. christian.cajochen@pukbasel.ch
Light can elicit acute physiological and alerting responses in humans, the magnitude of which depends on the timing, intensity, and duration of light exposure. Here, we report that the alerting response of light as well as its effects on thermoregulation and heart rate are also wavelength dependent. Exposure to 2 h of monochromatic light at 460 nm in the late evening induced a significantly greater melatonin suppression than occurred with 550-nm monochromatic light, concomitant with a significantly greater alerting response and increased core body temperature and heart rate ( approximately 2.8 x 10(13) photons/cm(2)/sec for each light treatment). Light diminished the distal-proximal skin temperature gradient, a measure of the degree of vasoconstriction, independent of wavelength. Nonclassical ocular photoreceptors with peak sensitivity around 460 nm have been found to regulate circadian rhythm function as measured by melatonin suppression and phase shifting. Our findings-that the sensitivity of the human alerting response to light and its thermoregulatory sequelae are blue-shifted relative to the three-cone visual photopic system-indicate an additional role for these novel photoreceptors in modifying human alertness, thermophysiology, and heart rate.
issue 2635 of New Scientist magazine, 26 December 2007, page 9
Curr Biol. 2007 Dec 18;17(24):2122-8.
Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina.
Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF 3rd, Czeisler CA, Foster RG, Moseley MJ, Lockley SW.
Division of Neuroscience and Mental Health, Faculty of Medicine, Imperial College London, London W6 8RF, United Kingdom.
As the ear has dual functions for audition and balance, the eye has a dual role in detecting light for a wide range of behavioral and physiological functions separate from sight. These responses are driven primarily by stimulation of photosensitive retinal ganglion cells (pRGCs) that are most sensitive to short-wavelength ( approximately 480 nm) blue light and remain functional in the absence of rods and cones. We examined the spectral sensitivity of non-image-forming responses in two profoundly blind subjects lacking functional rods and cones (one male, 56 yr old; one female, 87 yr old). In the male subject, we found that short-wavelength light preferentially suppressed melatonin, reset the circadian pacemaker, and directly enhanced alertness compared to 555 nm exposure, which is the peak sensitivity of the photopic visual system. In an action spectrum for pupillary constriction, the female subject exhibited a peak spectral sensitivity (lambda(max)) of 480 nm, matching that of the pRGCs but not that of the rods and cones. This subject was also able to correctly report a threshold short-wavelength stimulus ( approximately 480 nm) but not other wavelengths. Collectively these data show that pRGCs contribute to both circadian physiology and rudimentary visual awareness in humans and challenge the assumption that rod- and cone-based photoreception mediate all “visual” responses to light.
PMID: 18082405 [PubMed – in process]
issue 2567 of New Scientist magazine, 02 September 2006, page 40-43
Private communication April 7, 2008
issue 2649 of New Scientist magazine, 29 March 2008, page 23
Light isn’t just for vision anymore: implications for transportation safety.
Lighting Research Center, Rensselaer Polytechnic Institute Mariana G. Figueiro, Ph.D.
Tijdschr Gerontol Geriatr. 1998 Aug;29(4):177-84.
[Are active neurons a better defense against aging in Alzheimer’s disease?]
[Article in Dutch]
Lucassen PJ, van Someren EJ, Swaab DF.
Lucassen@LACDR.LeidenUniv.nl
This article deals with the question whether metabolic activity of neurons interferes with their survival during brain aging and Alzheimer’s disease (AD). This ‘use it or lose it’ concept assumes that active neurons have a better chance to survive these conditions. We have monitored activity changes in human hypothalamic nuclei, that show differential survival patterns in aging and AD. The size of the Golgi apparatus (GA) was measured in e.g. the nucleus basalis of Meynert (NBM), that is severely affected in AD, and in the vasopressin (AVP) containing neurons of the supraoptic nucleus (SON) that remain very stable and show no cell loss. In the affected NBM, a strong reduction in activity was found in AD, whereas in the stable SON, an increased activity was present in both conditions. These findings agree with the concept that activation is associated with pronounced stability in aging and AD. Another hypothalamic nucleus is the biological clock (SCN), which is very sensitive to light input. It loses about 35% of its AVP cells in old rats. In order to test the hypothesis that extra stimulation prevents degeneration, the SCN in old rats was activated by means of an increased light input. This could indeed prevent the age-related loss of AVP-neurons in the SCN in low light conditions. Increased light also restored the age-related decreased amplitude in the sleep-wake rhythm. Furthermore, in AD patients, increased amounts of environmental light improved day-night rhythms and reduced behavioural disturbances. These observations are in line with the ‘use it or lose it’ concept. Furthermore, oxidative damage to the DNA was studied as a) it may accumulate during neuronal aging, and b) activated cells repair their DNA more efficiently. Whereas biochemical measurements of 8OHDG levels were not different in aging or AD, in situ end labeling, that detects fragmented DNA histologically, showed many positive neurons and glial cells in the AD, but not control, hippocampus, whereas in SON and PVN, hardly any damage was detected, which agrees with the ‘use it or lose it’ concept. Supported by related literature, we conclude that activation may be effective for neuronal maintenance during aging and in AD, and may provide a fruitful basis in the search for future treatment strategies in AD.
: J Am Geriatr Soc. 2002 Feb;50(2):282-9.
Effect of light treatment on sleep and circadian rhythms in demented nursing home patients.
Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR.
Department of Psychiatry, University of California San Diego, USA. sancoliisrael@ucsd.edu
OBJECTIVES: To determine whether fragmented sleep in nursing home patients would improve with increased exposure to bright light. DESIGN: Randomized controlled trial. SETTING: Two San Diego-area nursing homes. PARTICIPANTS: Seventy-seven (58 women, 19 men) nursing home residents participated. Mean age +/- standard deviation was 85.7 +/- 7.3 (range 60-100) and mean Mini-Mental State Examination was 12.8 +/- 8.8 (range 0-30). INTERVENTIONS: Participants were assigned to one of four treatments: evening bright light, morning bright light, daytime sleep restriction, or evening dim red light. MEASUREMENTS: Improvement in nighttime sleep quality, daytime alertness, and circadian activity rhythm parameters. RESULTS: There were no improvements in nighttime sleep or daytime alertness in any of the treatment groups. Morning bright light delayed the peak of the activity rhythm (acrophase) and increased the mean activity level (mesor). In addition, subjects in the morning bright light group had improved activity rhythmicity during the 10 days of treatment. CONCLUSION: Increasing exposure to morning bright light delayed the acrophase of the activity rhythm and made the circadian rhythm more robust. These changes have the potential to be clinically beneficial because it may be easier to provide nursing care to patients whose circadian activity patterns are more socially acceptable.
PMID: 12028210 [PubMed – indexed for MEDLINE]
Int Psychogeriatr. 2005 Jun;17(2):221-36.
Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease.
Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJ.
University of California, San Francisco, Department of Physiological Nursing, 2 Koret Way, Room N631, San Francisco, CA 94143-0610, USA. glenna.dowling@nursing.ucsf.edu
BACKGROUND: Disturbances in rest-activity rhythm are prominent and disabling symptoms in Alzheimer’s disease (AD). Nighttime sleep is severely fragmented and daytime activity is disrupted by multiple napping episodes. In most institutional environments, light levels are very low and may not be sufficient to enable the circadian clock to entrain to the 24-hour day. The purpose of this randomized, placebo-controlled, clinical trial was to test the effectiveness of morning bright light therapy in reducing rest-activity (circadian) disruption in institutionalized patients with severe AD. METHOD: Subjects (n = 46, mean age 84 years) meeting the NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke–the Alzheimer’s Disease and Related Disorders Association) AD diagnostic criteria were recruited from two large, skilled nursing facilities in San Francisco, California. The experimental group received one hour (09:30-10:30) of bright light exposure (> or = 2500 lux in gaze direction) Monday through Friday for 10 weeks. The control group received usual indoor light (150-200 lux). Nighttime sleep efficiency, sleep time, wake time and number of awakenings and daytime wake time were assessed using actigraphy. Circadian rhythm parameters were also determined from the actigraphic data using cosinor analysis and nonparametric techniques. Repeated measures analysis of variance (ANOVA) was used to test the primary study hypotheses. RESULTS AND CONCLUSION: Although significant improvements were found in subjects with aberrant timing of their rest-activity rhythm, morning bright light exposure did not induce an overall improvement in measures of sleep or the rest-activity in all treated as compared to control subjects. The results indicate that only subjects with the most impaired rest-activity rhythm respond significantly and positively to a brief (one hour) light intervention.
PMID: 16050432 [PubMed – indexed for MEDLINE]
J Am Geriatr Soc. 2008 Feb;56(2):239-46. Epub 2007 Dec 7.
Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease.
Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA.
Department of Physiological Nursing, University of California at San Francisco, San Francisco, California 94143, USA. glenna.dowling@nursing.ucsf.edu
OBJECTIVES: To test whether the addition of melatonin to bright-light therapy enhances the efficacy in treating rest-activity (circadian) disruption in institutionalized patients with Alzheimer’s disease (AD). DESIGN: Randomized, controlled trial. SETTING: Two nursing homes in San Francisco, California. PARTICIPANTS: Fifty subjects (mean age 86) with AD. INTERVENTION: Experimental subjects received 1 hour of morning light exposure (> or = 2,500 lux in gaze direction) Monday to Friday for 10 weeks and 5 mg melatonin (LM, n=16) or placebo (LP, n=17) in the evening. Control subjects (n=17) received usual indoor light (150-200 lux). MEASUREMENTS: Nighttime sleep variables, day sleep time, day activity, day:night sleep ratio, and rest-activity parameters were determined using actigraphy. RESULTS: Linear mixed models were employed to test the primary study hypotheses. No significant differences in nighttime sleep variables were found between groups. At the end of the intervention, the LM group showed significant improvement in daytime somnolence as indicated by a reduction in the duration of daytime sleep, an increase in daytime activity, and an improvement in day:night sleep ratio. The LM group also evidenced a significant increase in rest-activity rhythm amplitude and goodness of fit to the cosinor model. CONCLUSION: Light treatment alone did not improve nighttime sleep, daytime wake, or rest-activity rhythm. Light treatment plus melatonin increased daytime wake time and activity levels and strengthened the rest-activity rhythm. Future studies should resolve the question of whether these improvements can be attributed to melatonin or whether the two zeitgebers interact to amplify efficacy.
Biol Psychiatry. 1998 Jun 1;43(11):822-8.Click here to read Links
Treatment of rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleep.
Wehr TA, Turner EH, Shimada JM, Lowe CH, Barker C, Leibenluft E.
Clinical Psychobiology Branch, National Institute of Mental Health, Bethesda, Maryland 20892-1390, USA.
BACKGROUND: The modern practice of using artificial light to extend waking activities into the nighttime hours might be expected to precipitate or exacerbate bipolar illness, because it has been shown that modifying the timing and duration of sleep can induce mania in susceptible individuals. With this possibility in mind, we treated a patient with rapidly cycling bipolar illness by creating an environment that was likely to increase and to stabilize the number of hours that he slept each night. METHODS: We asked the patient to remain at bed rest in the dark for 14 hours each night (later this was gradually reduced to 10 hours). Over a period of several years, his clinical state was assessed with twice-daily self-ratings, once-weekly observer ratings, and continuous wrist motor activity recordings. Times of sleeping and waking were recorded with sleep logs, polygraphic recordings, and computer-based event recordings. RESULTS: The patient cycled rapidly between depression and mania and experienced marked fluctuations in the timing and duration of sleep when he slept according to his usual routine, but his sleep and mood stabilized when he adhered to a regimen of long nightly periods of enforced bed rest in the dark. CONCLUSIONS: Fostering sleep and stabilizing its timing by scheduling regular nightly periods of enforced bed rest in the dark may help to prevent mania and rapid cycling in bipolar patients.
Biol Psychiatry. 1999 Apr 15;45(8):1075-7.Click here to read Links
A rapid-cycling bipolar patient treated with long nights, bedrest, and light.
Wirz-Justice A, Quinto C, Cajochen C, Werth E, Hock C.
Psychiatric University Clinic, Basel, Switzerland.
BACKGROUND: Stabilization of rapid-cycling bipolar disorder is extremely difficult. METHODS: A refractory bipolar I rapid-cycling patient on valproate was treated with long “nights” (extended sleep in darkness) and daytime light therapy. RESULTS: Rapid cycling immediately stopped on initiation of a 10 hour dark/rest period. This was extended to 14 hours (plus a self-selected 1 hour midday nap) without problems. Depression gradually improved when midday light therapy was added; near-euthymia was attained after light therapy was shifted to the morning. CONCLUSIONS: Nonpharmacological chronobiological treatments may be a means to interrupt rapid cycling.
Bipolar Disord. 2005 Feb;7(1):98-101.
Dark therapy for mania: a pilot study.
Barbini B, Benedetti F, Colombo C, Dotoli D, Bernasconi A, Cigala-Fulgosi M, Florita M, Smeraldi E.
Department of Neuropsychiatric Sciences, Istituto Scientifico Universitario Ospedale San Raffaele, Milano, Italy.
BACKGROUND: Recent findings suggest that extended bed rest and darkness could stabilize mood swings in rapid cycling bipolar patients. METHOD: We exposed 16 bipolar inpatients affected by a manic episode to a regimen of 14 h of enforced darkness from 6 p.m. to 8 a.m. each night for three consecutive days [dark therapy (DT)]. Pattern of mood changes were recorded with the Young Mania Rating Scale (YMRS) and compared with a control group of 16 inpatients matched for age, sex, age at onset, number of previous illness episodes and duration of current episode, and were treated with therapy as usual (TAU). RESULTS: Adding DT to TAU resulted in a significantly faster decrease of YMRS scores when patients were treated within 2 weeks from the onset of the current manic episode. When duration of current episode was longer, DT had no effect. Follow-up confirmed that good responders needed a lower dose of antimanic drugs and were discharged earlier from the hospital. CONCLUSIONS: Chronobiological interventions and control of environmental stimuli can be a useful add-on for the treatment of acute mania in a hospital setting. Copyright (c) 2005, Blackwell Munksgaard. http://www.denvernaturopathic.com/bluelightandmelatonin.htm





